We examine the viability of cells by trypan blue staining after thawing of frozen vials. In addition, we check the adhesion efficiency after overnight culture for the adherent cells.
1.Preparation
Frozen Cells
Medium
Centrifuge tube
Basic cell culture equipment
Trypan blue staining solution
Hemocytometer
2.Method
2-2. Frozen cells should be thawed quickly using 37℃ water bath
2-3. Gently pippet cell suspension and transfer to the tube containing medium
2-4. Centrifuge 1,000 RPM at room temperature for 3 mins and discard the supernatant (repeat twice this step)
2-5. Add 5 ml medium and suspend cell pellet
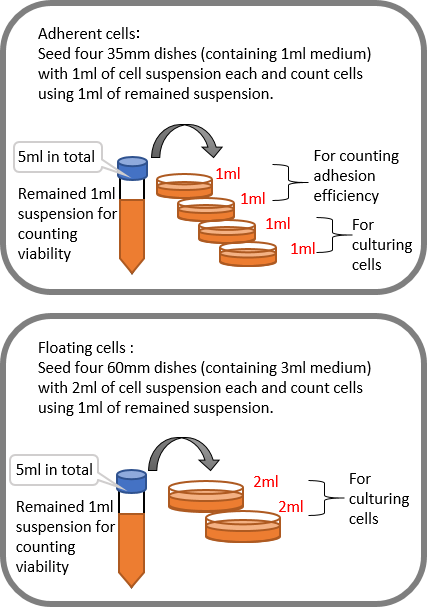
2-6. Plate cells as shown at right in the picture
2-7. Measure cell viability
| Cell Viability (%) = B x 5 / A x 100 |
Count viable cells by staining cell suspension with trypan blue.
A (Cells/tube) = Frozen viable cell number per frozen vial
*Please refer to the data sheet enclosed with cells.
B (Cells/ml) = Viable cell number in 1 ml of cell suspension
Our bank routinely examine the cells from randomly selected two frozen vials, and measure cell viability and adhesion efficiency by their average values.
2-8. Measure adhesion efficiency on the next day (in case of adherent cells)
| Adhesion efficiency (%) = C / B x 100 |
Collect cells from two 35 mm dishes out of four dishes and stain them,
then count viable cells of each dish.
C (cells / 35 mm dish) = The average value of viable cells in two dishes





